Author
Author  Correspondence author
Correspondence author
Cancer Genetics and Epigenetics, 2024, Vol. 12, No. 3
Received: 12 Apr., 2024 Accepted: 19 May, 2024 Published: 31 May, 2024
The core concept of personalized treatment is to develop individualized treatment strategies based on the unique characteristics of the patient and the molecular features of the tumor, aiming to provide more effective and less toxic treatment options. The rapid advancement of precision medicine technologies has strongly supported the implementation of personalized treatment, including the association between gene mutations and cancer development, the application of genomics technologies, and the identification of tumor driver genes. This review comprehensively discusses the definitions and development trends of personalized and precision cancer treatment, the application of molecular biology and genomics in cancer research, precise methods for diagnosis and staging, and personalized treatment approaches and applications. It also emphasizes the importance of personalized and precision treatment in cancer therapy, encouraging future research and clinical practice to offer better treatment options for patients, advance the field, and ultimately reduce cancer mortality.
Cancer has long been a major challenge in the global health sector, not only due to its high incidence and mortality rates but also because of its high heterogeneity. Different types and subtypes of cancer exhibit significant differences at the molecular level, making traditional cancer treatments inadequate for meeting the needs of all patients. However, with rapid advancements in the biomedical field, particularly in molecular biology, genomics, and bioinformatics, personalized and precision therapies are emerging in cancer treatment.
Personalized treatment refers to selecting the most appropriate treatment strategy based on an individual's genotype, phenotype, lifestyle, and environmental factors (Gambardella et al., 2020). This means that different patients may receive different treatment regimens tailored to better address their unique cancer characteristics. Compared to traditional cancer treatments, personalized treatment is more precise and effective, reducing adverse reactions and side effects during the treatment process.
Precision medicine focuses more on using molecular biology and genomics technologies to select the most suitable treatment strategy by thoroughly understanding the molecular characteristics of a patient's tumor (Tsimberidou et al., 2020). This approach enables doctors to provide highly individualized treatment plans, increasing the chances of successful outcomes. By understanding the molecular features of a patient's tumor, doctors can choose targeted therapies, immunotherapies, or other patient-specific treatments, thereby minimizing ineffective attempts during the treatment process.
Therefore, personalized and precision therapies have become the forefront of cancer treatment. They not only provide better treatment options for patients but also play a crucial role in improving treatment outcomes and survival rates. This review delves into the concepts, technologies, and applications of these therapies, offering insights into how they are transforming cancer treatment and further advancing the development of personalized and precision therapies.
1 Epidemiology and Development Trends of Cancer
1.1 Global epidemiology of cancer
Cancer is a significant global health challenge, with millions of people diagnosed with various types of cancer each year. Global epidemiological studies on cancer aim to understand the distribution of cancer incidence and mortality rates across different regions, populations, and age groups. There are noticeable disparities in cancer incidence and mortality between different regions. Developed countries typically report higher cancer incidence rates, while developing countries face higher cancer mortality rates. Additionally, the prevalence of different types of cancer varies by region. Lung cancer, breast cancer, prostate cancer, and colorectal cancer are among the most common cancers worldwide (Yuan et al., 2021). Such studies help reveal the impact of various risk factors, such as smoking, diet, environmental factors, and genetic predispositions, on cancer incidence. Global epidemiological data also provide crucial information for guiding health policies and resource allocation to better prevent and treat cancer.
1.2 Types and classification of cancer
Cancer is a diverse group of diseases with complex types and classifications. Essentially, cancers are classified based on the tissues or organs where they originate. For instance, breast cancer originates in breast tissue, while lung cancer originates in lung tissue. Different types of cancer typically exhibit distinct growth patterns and pathological characteristics, which can be determined through tissue biopsies and pathological examinations. Detailed histological classification allows doctors to identify the specific type of cancer a patient has, aiding in the selection of the most appropriate treatment strategy.
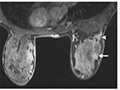
Moreover, cancer can be further classified based on cell type, tissue origin, and molecular characteristics. For example, breast cancer can be subdivided into different subtypes such as hormone receptor-positive and HER2-positive breast cancer (Figure 1), which have different treatment options. Similarly, lung cancer can be divided into small cell lung cancer and non-small cell lung cancer, each with distinct growth patterns and treatment approaches. The detailed classification of cancer helps doctors better understand the nature of the disease, enabling the development of more individualized treatment plans.
|
Figure 1 Acoustic image of HER2 positive breast cancer |
1.3 Trends in Cancer Development
The trends in cancer development are continually evolving, influenced by various factors. Firstly, demographic changes, especially the increase in the aging population, have led to a rise in the incidence of certain cancers. Additionally, lifestyle factors such as smoking, diet, and physical activity significantly impact cancer risk. For example, there is a strong association between smoking and lung cancer (Wang et al., 2022), while a high-fiber diet is linked to a reduced risk of colorectal cancer.
On the other hand, improvements in early cancer screening and treatment methods have significantly impacted the incidence and mortality rates of certain cancers. Early cancer screening allows for the detection of tumors at the early stages of disease development, thereby increasing the chances of successful treatment. Moreover, new treatment methods, such as immunotherapy and targeted therapy, offer better treatment options for some cancer patients. The advancement of these treatment modalities is changing the trends in cancer development, leading to improved survival rates for patients.
2 Concepts of Personalized Medicine and Precision Medicine
2.1 Definition of personalized medicine
Personalized medicine, also known as individualized medicine, is a medical approach tailored to the unique characteristics of each patient based on their genetic, molecular, physiological, and clinical features. This medical model aims to individualize medical decisions and treatment plans to better meet the specific needs of each patient. The core concept of personalized medicine is that every person is unique, and their diseases and health conditions should be treated in a unique way. This means that medical decisions are no longer solely based on general standards and guidelines but are instead crafted according to the individual characteristics of each patient, thereby enhancing treatment efficacy and minimizing unnecessary side effects.
2.2 Definition of precision medicine
Precision medicine, also known as individualized medicine, is a specific practice of personalized medicine that focuses on using advanced molecular and genetic technologies to gain a more precise understanding of each patient’s biological characteristics, including their genome, proteome, and metabolome. The goal of precision medicine is to accurately diagnose diseases, predict disease risk, determine the most appropriate treatment plans, and monitor treatment outcomes. It emphasizes the precise measurement and deep understanding of individual differences to better match treatment methods to disease characteristics. Precision medicine relies on advanced molecular biology and bioinformatics tools to provide more comprehensive and accurate medical care (Tsimberidou et al., 2020).
2.3 Relationship between personalized medicine and precision medicine
Personalized medicine and precision medicine are closely related concepts that both aim to better meet the individualized needs of patients. In fact, precision medicine can be seen as a means to achieve personalized medicine. Personalized medicine focuses on providing individualized medical services and treatment plans, while precision medicine provides the scientific and technological foundation necessary to achieve this goal. By deeply researching patients' biological characteristics, such as genotypes and biomarkers, precision medicine enables doctors to formulate more accurate and individualized treatment plans. Therefore, these two concepts are closely intertwined, jointly driving the development of the modern medical field and providing more effective medical care for patients.
3 Application of Molecular Biology and Genomics in Cancer Research
3.1 Gene mutations and cancer development
The development of cancer is closely associated with gene mutations. Gene mutations refer to changes in the DNA sequence of the genome, which may alter the function of genes. In many cases, the origin of cancer can be traced back to mutations in certain key genes (Liang et al., 2020). These genes can be categorized into two types: tumor suppressor genes and oncogenes. Tumor suppressor genes normally function to inhibit uncontrolled cell growth, but when they mutate, cells lose this inhibitory effect, leading to the formation of cancer cells. Conversely, mutations in oncogenes result in their overactivation, promoting abnormal cell proliferation. Through molecular biology techniques, researchers can identify mutations in these critical genes, thereby gaining a better understanding of the mechanisms underlying cancer development.
3.2 Application of genomic technologies
In recent years, the rapid development of genomic technologies has become an essential tool in cancer research. Techniques such as whole-genome sequencing, exome sequencing, and RNA sequencing allow scientists to comprehensively analyze the genetic information of tumor cells. These technologies enable researchers to identify various gene mutations in tumors, including single nucleotide polymorphisms, copy number variations, and chromosomal rearrangements. Additionally, RNA sequencing allows for the analysis of gene expression patterns in tumor cells (Zhang et al., 2023), providing insights into which genes play critical roles in the onset and progression of cancer. The application of these genomic technologies offers valuable information for the precise diagnosis and treatment of cancer.
3.3 Identification of tumor driver genes
Tumor driver genes are genes that play key roles in the development of cancer. These genes are typically mutated, leading to abnormal cell proliferation and survival. Through molecular biology and genomic technologies, researchers can identify these tumor driver genes. Once identified, these genes become potential therapeutic targets. Some drugs have already been developed to target specific tumor driver genes, thereby inhibiting tumor growth. This personalized treatment strategy has achieved significant success in clinical settings, providing better treatment options for cancer patients.
4 Precision in Cancer Diagnosis and Staging
4.1 Early cancer diagnosis methods
Early cancer diagnosis has always been a significant challenge in the medical field because, in the early stages of cancer, there are usually no apparent symptoms or signs, leading to cancers often being detected at advanced stages. However, early cancer diagnosis is crucial for treatment and survival rates since, at this stage, the cancer has typically not spread to other tissues or organs, and the chances of successful treatment are higher.
In the context of personalized and precision medicine, researchers and doctors continually strive to improve early cancer diagnosis methods. Tumor markers are a class of biomolecules, such as specific proteins or metabolites, that typically change in the blood or bodily fluids of cancer patients (Faria et al., 2019). By detecting the levels of these markers, doctors can suspect the presence of cancer. Common tumor markers include PSA (prostate-specific antigen), CA-125 (ovarian cancer marker), and CEA (carcinoembryonic antigen). Although tumor markers can provide clues, they are not 100% specific and sensitive, so other tests are usually needed to confirm the diagnosis.
Medical imaging technologies such as computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET-CT) (Figure 2) play a crucial role in early cancer diagnosis. These technologies can be used to detect the size, location, and spread of tumors, helping doctors determine whether the tumor is in its early stage. With continuous technological advancements, the accuracy of imaging in early cancer diagnosis has significantly improved.
 Figure 2 Positron Emission and Computer Tomography System |
In recent years, the application of genomic technologies has made it possible to diagnose early-stage cancers by detecting circulating tumor DNA (ctDNA) or circulating tumor cells (CTC). These technologies allow doctors to detect genetic changes in tumors from blood samples, enabling diagnosis even when the tumors are very small or in early stages. This non-invasive approach shows great promise for early cancer screening and diagnosis. Tissue biopsy, a method of directly collecting tissue samples from patients, is typically performed via endoscopy or surgery. This method is usually employed to determine the type and pathological characteristics of the tumor. For early-stage cancers, tissue biopsy is often conducted when a tumor is suspected to confirm the diagnosis.
4.2 Application of molecular markers
Molecular markers are molecules or biological markers used to identify disease states. In the field of cancer, molecular markers can aid in diagnosis, staging, and predicting patient prognosis. These markers can include proteins, nucleic acids, or metabolites. By analyzing molecular markers in tumor samples, doctors can gain a better understanding of the disease and develop precise treatment plans based on the individual characteristics of the patient. The application of molecular markers plays a crucial role in cancer research and clinical practice.
4.3 Role of imaging technologies in diagnosis
Imaging technologies such as CT, MRI, and PET-CT play a significant role in the diagnosis and staging of cancer. These technologies can be used to detect the location, size, and spread of tumors. With continuous technological advancements, the precision of imaging in cancer diagnosis has significantly improved. Additionally, the integration of molecular markers with medical imaging is expected to become a more accurate diagnostic method, thereby better guiding treatment decisions.
5 Personalized Treatment Methods and Applications
5.1 Targeted therapy and targeted drugs
A key aspect of personalized treatment is targeted therapy, which involves selecting appropriate drugs based on the molecular characteristics of a patient's tumor. The fundamental concept of targeted therapy is to selectively kill or inhibit cancer cells by interfering with their growth and proliferation mechanisms, while minimizing damage to normal cells. This approach is more individualized and precise compared to traditional radiotherapy and chemotherapy.
Targeted drugs are specifically designed to interfere with particular molecules or signaling pathways in cancer cells. These drugs typically work by inhibiting or intervening in molecular targets, such as kinases or receptor proteins (Zhong et al., 2021). For example, for breast cancer patients expressing HER2 (human epidermal growth factor receptor 2), drugs like Herceptin (trastuzumab) can target this receptor, effectively treating the cancer. Therefore, targeted therapy relies on a deep understanding of the molecular characteristics of the patient's tumor, often requiring genetic testing or protein analysis to determine the most suitable drugs.
5.2 Immunotherapy
Immunotherapy is another crucial personalized treatment approach that utilizes the patient's immune system to recognize and attack cancer cells. The core concept of immunotherapy is to enhance or reactivate the patient's immune response to inhibit tumor growth and spread (Xu et al., 2021). The importance of this method lies in the fact that the immune response varies among patients, necessitating individualized treatment strategies based on the patient's immune characteristics.
Applications of immunotherapy include immune checkpoint inhibitors, such as anti-PD-1 and anti-CTLA-4 drugs, which prevent cancer cells from evading immune surveillance. CAR-T cell therapy is another innovative immunotherapy approach involving the extraction of the patient's T cells, genetic modification to enhance their anti-cancer capabilities, and reinfusion into the patient. The selection and adjustment of these immunotherapies require personalization based on the patient's immune status and tumor characteristics.
5.3 Gene therapy and gene editing technologies
Gene therapy and gene editing technologies are at the forefront of personalized treatment, directly intervening in the patient's genes or gene expression to treat cancer. One application of gene therapy involves introducing genes with anti-cancer functions into the patient to enhance their ability to combat cancer. Gene editing technologies allow for the modification or repair of abnormal genes in the patient, thereby preventing tumor development.
For example, gene therapy can be developed to introduce anti-cancer genes such as the p53 gene, helping the patient's immune system to better attack cancer cells. Gene editing technologies like CRISPR-Cas9 have been used to repair cancer-related gene mutations, reducing the patient's cancer risk. These advanced techniques hold great promise for the future of personalized cancer treatment, offering precise and effective options tailored to the genetic profile of each patient.
6 Conclusion and Outlook
This review has comprehensively discussed the importance of personalized and precision medicine in cancer treatment and its applications. It highlights that the core concept of personalized cancer treatment is to develop individualized treatment strategies based on the unique characteristics of the patient and the molecular features of the tumor. This approach provides more effective and less toxic treatment options for patients. The rapid development of precision medicine technologies, such as molecular biology, genomics, immunotherapy, and gene editing, has strongly supported the implementation of personalized treatment. Personalized and precision treatments are crucial in cancer therapy. Traditional treatment methods, although effective, often come with severe side effects, and their efficacy varies among patients. Personalized treatment shifts the strategy from a "one-size-fits-all" approach to an individualized one, better meeting patients' needs, increasing treatment success rates, and reducing unnecessary toxic side effects.
To further advance personalized and precision treatments, more research and clinical practice are needed. Researchers should continue to explore the relationship between tumor molecular characteristics and treatment responses, develop new treatment strategies, and conduct more clinical trials to verify the efficacy and safety of these methods. Additionally, interdisciplinary collaboration will play a key role, combining molecular biology, medicine, computer science, and clinical practice to drive the realization of personalized treatment.
In the future, personalized and precision treatments will continue to be a major trend in cancer research and therapy. With continuous advancements in molecular biology and genomics, we can gain deeper insights into the molecular mechanisms of tumors, discover new therapeutic targets, and develop more precise treatment methods. Moreover, artificial intelligence and big data analysis will play an increasingly significant role in personalized treatment, helping doctors better predict patients' treatment responses.
The prospects for personalized and precision treatments are very promising. By better understanding patients' genetic and molecular characteristics, doctors can create unique treatment plans for each patient, offering better treatment outcomes and survival rates. Over time, we can expect more types of cancer to have targeted treatment strategies, with patients benefiting from fewer side effects and higher treatment success rates.
Acknowledgments
I would like to express my gratitude to Ms. Han for her valuable suggestions from the selection of the research topic to the completion of the project.
Conflict of Interest Disclosure
The author affirms that this research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Faria S.C., Sagebiel T., Patnana M., Cox V., Viswanathan C., Lall C., Qayyum A., and Bhosale P.R., 2019, Tumor markers: myths and facts unfolded, Abdominal Radiology, 44: 1575-1600.
https://doi.org/10.1007/s00261-018-1845-0
PMid:30498924
Gambardella V., Tarazona N., Cejalvo J.M., Lombardi P., Huerta M., Roselló S., and Cervantes A., 2020, Personalized medicine: recent progress in cancer therapy, Cancers, 12(4): 1009.
https://doi.org/10.3390/cancers12041009
PMid:32325878 PMCid:PMC7226371
Huang Z.M., Zeng F., Wei W.L., Zhang Y., and Zhao J.M., Research on the application of artificial intelligence in clinical teaching in the context of new medical science, Chuanxin Jiaoyu Yanjiu (Creative Education Studies), 11(10): 3066-3072.
https://doi.org/10.12677/CES.2023.1110452
Liang B., Ding H., Huang L., Luo H., and Zhu X., 2020, GWAS in cancer: progress and challenges, Molecular Genetics and Genomics, 295: 537-561.
https://doi.org/10.1007/s00438-020-01647-z
Tsimberidou A.M., Fountzilas E., Nikanjam M., and Kurzrock R., 2020, Review of precision cancer medicine: evolution of the treatment paradigm, Cancer Treatment Reviews, 86: 102019.
https://doi.org/10.1016/j.ctrv.2020.102019
PMid:32251926 PMCid:PMC7272286
Wang H., Wei X.X., Ma Z.M., Ji M.M., Huang Y.Q., Zhang J., Zhu M., Dai J.C., Jin G.F., Ma H.X., Hu Z.B., and Shen H.B., 2022, Mediation effect of smoking and healthy diet score on the association between educational level and the risk of lung cancer incidence, Zhonghua Liuxingbingxue Zazhi (Chinese Journal of Epidemiology), 43(12): 1875-1880.
Xu D., Lu L., and Qin L.X., 2021, Recent progress in precision immunotherapy of hepatocellular carcinoma, Zhongliu Zhonghe Zhiliao Zazhi (Journal of Multidisciplinary Cancer Management(Electronic Version)), 7(3): 6-11.
Yuan H.Y., Jiang Y.F., Tan Y.T., and Xiang Y.B., 2021, Current status and time trends of cancer incidence and mortality worldwide, Zhongliu Fangzhi Yanjiu (Cancer Research on Prevention and Treatment), 48(6): 642-646
Zhang W.Q., Tian S.N., and Feng Z.Q., Applications of single cell RNA sequencing technology in the study of non-small cell lung cancer, Nanjing Yike Daxue Xuebao (Journal of Nanjing Medical University(Natural Sciences)), (6): 864-870.
Zhong L., Li Y., Xiong L., Wang W., Wu M., Yuan T., Yang W., Tian C.Y., Miao Z., Wang T.Q., and Yang S., 2021, Small molecules in targeted cancer therapy: Advances, challenges, and future perspectives, Signal Transduction and Targeted Therapy, 6(1): 201.
https://doi.org/10.1038/s41392-021-00572-w
PMid:34054126 PMCid:PMC8165101
(1).png)
. FPDF(win)
. FPDF(mac)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Jian Wang
Related articles
. Cancer
. Personalized treatment
. Precision medicine
. Molecular biology
. Genomics
Tools
. Post a comment